
Date:
Location:
Speaker:
ABSTRACT:
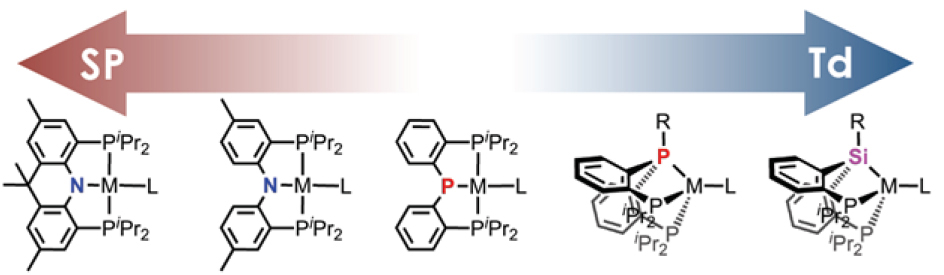
Transition metal adduct formations with small molecules such as N2, H2, CO and CO2 are drawing much attention due to their importance in developing synthetic catalysts for various industrial processes. The chemistry is based on pincer complexes with attention to the uniqueness of the coordination geometry (square-planar or pseudotetrahedral), which is crucial in allowing for particular reactivity toward small molecules. In our laboratory, a series of pincer complexes with low-valent1st row transition metals are currently under investigation. Synthesis and characterization of four coordinate (PEP)M-L complexes (E = N or P and M = Co, Ni) will be described, where the L site is occupied by various ligands such as NHR2, N2, COxand COOR. Regarding the geometry and reactivity relationship, a (PPP)M scaffold reveals the interconversion between square planar and tetrahedral geometry, in which reversible alkoxygroup transfer occurs between a phosphide moiety of a PPP ligand and a nickel ion via unanticipated metal-ligand cooperation. This unusual group transfer reaction is tightly coupled with metal’s local geometry and its 0/II redox couple. In fact, a central phosphide moiety of a PPP ligand acts as a single electron donor to form a P radical revealing the metal-ligand cooperativity involving a single electron exchange between Ni and P. By employing such cooperativity, nitrenegroup transfer was successfully accomplished to generate a dimeric nickel(0)-CO species along with mesitylamineand mesitylisocyanate. In contrast, a (PNP)M scaffold presents unusual reactivity occurring at the structurally rigidified nickel center. Unique open-shell reactivity of a T-shaped nickel(I) metalloradicalsupported by a rigidified acridane-based pincer ligand will be discussed. Having a sterically exposed half-filled dx2-y2 orbital, this three-coordinate NiIspecies reveals unique open-shell reactivity including the homolyticcleavage of various -bonds, such as H-H, N-N, and C-C.
BIO:
Yunho Lee is a tenured associate professor in the Department of Chemistry at Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea. He attended ChonbukNational University where he received a B.S. degree in Chemistry in 2000. Yunho left Korea to begin his doctoral studies under the guidance of Prof. Kenneth D. Karlinat the Johns Hopkins University, Baltimore. After receiving his Ph.D. in 2007, Yunho was a postdoctoral fellow in the laboratory of Prof. Jonas C. Peters at the Massachusetts Institute of Technology and the California Institute of Technology. In the winter of 2010, Yunho returned to Korea and started his independent career as an Assistant Professor at KAIST. He was promoted to associate professor in September 2015. His primary research interests focus on the fundamental transition metal coordination chemistry and its application in various small molecule conversions and catalysis inspired by metalloenzyme. Selected awards include the Distinguished Lectureship Award from the Chemical Society of Japan (2011), the Young Inorganic Chemist Award (2015) and KCS-Wiley Young Chemist Award (2017) from the Korea Chemical Society.



